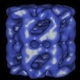
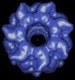
GroEL Dataset I » History » Revision 10
« Previous |
Revision 10/31
(diff)
| Next »
Eric Hou, 07/19/2010 03:32 PM
Anontated Dataset I of Images of GroEL Particles¶
1. Imaging Conditions¶
Using a FEI Tecnai F20 equipped with a 2Kx2K CCD Tietz camera, images are acquired in defocus pairs at a nominal magnification of 62,000 x and a voltage of 120 KeV, using the Leginon system (Carragher et al., 2000). The first image (named *.001.mrc) is acquired at very near to focus (NTF) conditions (-0.6 to -1.5 µm) and the second one (named *.002.mrc) at farther from focus (FFF) conditions (-2 µm). The time interval between the two exposures is approximately 20s due to the time required to read out the digital image from the camera. The pixel size is 2.238 Å at the specimen scale, and the accumulated dose for high magnification NTF image area was about 10 e/Ų. Figure 1 shows an example pair of defocus images. Click on them to see the pictures in full size.
(a) 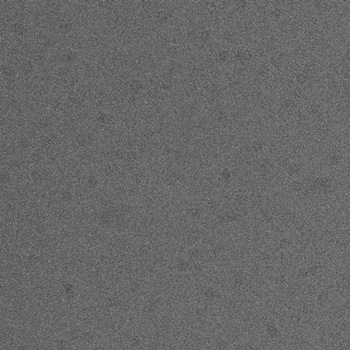 (b)
(b) 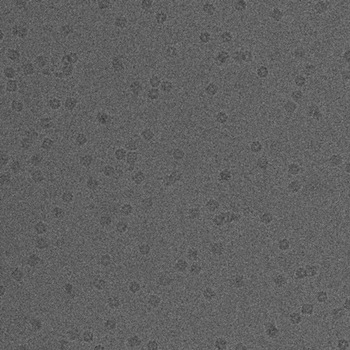
(a) Near to focus (NTF) image. (b) Far from focus - FFF image.
Figure 1: An example pair of high magnification images of GroEL.
There are at least two major advantages of using a defocus pair of images. First, by combining the two images in the defocus pair, relatively high contrast at both low and high spatial frequencies can be attained. Second, the moderately strong low-resolution signals in the FFF images make it possible for us to develop algorithms to identify particles automatically.
2. Downloading High Magnification Images¶
Image files are in MRC format. We also provide a JPEG file for each image for your convenience in viewing them. The dataset consists of 384 pairs of high magnification images acquired on October 9, 2003 in a single experiment session. You may use one of the following options to download the set of high magnification images.
Download the whole experiment dataset using our download wizard, called dbemwiz.jar. Here are a few tips for using the wizard to download the whole set of high magnification images: (i) Log in as anonymous. (ii) From the list of Experiments select "03oct09b", shown in Fig. 2a, which is the experiment identification used in our Leginon database. (iii)Select "exposures" for Image Type as shown in Figure 2b and then proceed to downloading.
(a)  (b)
(b) 
Figure 2: Screenshots of using the wizard with the above tips to download the whole set of high magnification images.
3. Coordinates of the Picked Particles in the Images¶
Since the NTF image in a defocus pair covers almost the same specimen area as the FFF image, the relative distance between particles within the NTF image should be the same as that in the FFF image. Using phase correlation, we are able to accurately align the NTF image to the FFF image in a defocus pair (Zhu et al., 2001). Therefore particles in the NTF image can be then extracted using the positions of the particles selected in the FFF image shifted according to the results of the alignment. We provide in the following only the positions of particles automatically selected in the FFF images.
(a)  (b)
(b) 
(a) The NTF image. (b) The FFF image.
Figure 3: An example pair of images outlined with particles selected by Selexon. Each "+" indicate a detected particle.
As we mentioned in the introduction, particle picking is an open, unresolved problem. Even for biological experts, the final picks may vary from person to person. Besides posting the particles picked by our own program Selexon, we will also post other manual/automated picks upon available. For each set of picked particles, we give a brief description of the criteria for manual picks, or the algorithm for automated picks. Links to more detailed descriptions will be provided when available.
Table 1: Manually or automatically picked particles.
| Picker | Brief Description of the Algorithm | Links to download |
| Selexon | A two-stage approach was applied for automatic selection of GroEL particles. In the first stage, a set of candidate particles were automatically selected for each micrograph using the fast local correlation algorithm (Roseman, 2003) combined with our own peak searching and thresholding algorithms. In the second stage, the set of candidate particles were filtered with a clutter finder to reject any contaminations (Zhu et al., 2004). The clutter finder starts with edge detection followed by connected componnet labeling, convex hull computation, and size filtering. | List of 16212 side-view and that of 10603 top-view GroEL particles selected in the FFF images. |
4. Sample 3D Reconstructions¶
Table 2: Sample reconstruction using 7,505 side-view particles.
5. References ¶
- Carragher, B., Kisseberth, N., Kriegman, D., Milligan, R. A., Potter, C. S., Pulokas, J., and Reilein, A. (2000) Leginon: An automated system for acquisition of images from vitreous ice specimens. J. Struct. Biol. 132: 33-45.
- Ludtke, S.J., P.R. Baldwin, and W. Chiu. (1999) EMAN: Semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128: 82-97.
- Roseman, A.M. (2003) Particle finding in electron micrographs using a fast local correlation algorithm. Ultramicroscopy 94: 225-236.
- Zhu, Y., B. Carragher, D.J. Kriegman, R. Milligan, and C.S. Potter. (2001) Automated identification of filaments in cryo-electron microscopy images. J. Struct. Biol. 135: 302-312.
- Zhu, Y., Carragher, B., and Potter, C. S. (2004) Contaminant detection: improving tempalte matching based particle selection for cryo-electron microscopy. In: Proceedings of IEEE International Symposium on Biomedical Imaging, pp. 1071-1074, April 15-18, 2004, Arlington, VA.
Updated by Eric Hou over 15 years ago · 31 revisions